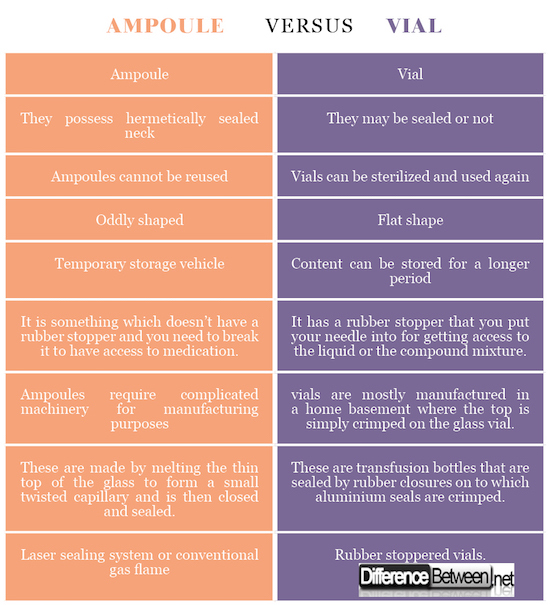
Types Of Rubber Stoppers For Pharmaceuticals Pdf

It is an object of the present invention to provide.
Types of rubber stoppers for pharmaceuticals pdf. The mixing of raw materials and auxiliary substances involves the formulation of the stopper. A determination was made of various chemical compounds leached from rubber stoppers into large volume parenteral solutions. A rubber stopper composition that is easy to mold and process excellent in sealing properties and gas barrier properties low in propensity to elute into the contents and satisfactory in needle penetration. From rubber trees natural rubber latex is collected after processed and the main standard piece of rubber and rubber smoke is crafted.
The number of particles released into the sodium chloride solution was measured by. All rubber stoppers are stored in and supplied from our iso certified warehouse in uithoorn. West stoppers protect drugs from environmental impact and help maintain quality and safety. The effect of sterilization on the number of particles released from five different types of rubber stoppers as well as on their surface roughness and elemental composition before and after sterilization is described.
With the exception of furfural the same chemical compounds were extracted from the parenteral solutions and from unused stoppers. á381ñ elastomeric closures for injections introduction elastomeric closures for containers used in the types of preparations defined in the general test chapter injections and im planted drug products á1ñ are made of materials obtained by vulcanization cross linking polymerization polyaddition or poly condensation of macromolecular organic substances elastomers. The film coated rubber stopper reduces the closure drug interaction thereby reducing the amount of leachables in the drug product. A stopper is typically made up of 60 rubber 30 fillers which protect the physical properties of the rubber and pigments 5 plasticizers which provide flexibility 5 additional chemicals including accelerators which help to create the cross linkages which give the stopper its strength and.
The storage forwarding and delivery of the rubber stoppers takes place under conditions that are consistent with the pharmaceutical guidelines and good distribution practice gdp. Visit our online store. Lower particle limits should be introduced to reflect modern needs. Novapure stoppers west ready pack system 4040 40 formulation.
Examples are given below. And a medical rubber stopper made by using the same. To go into the human body as a result pharmaceutical stoppers must be no abnormal toxicity no heat nonhemolytic reactions so as to make sure the safety and security of using medications. This has rightly become a common concern with the us fda and is a.
The stoppers were immersed in 200 ml of 0 9 sodium chloride solution in conical flasks. At apg pharma it s all about quality expertise and quality care. The object of the present invention is attained by a composition made up in a. There are several differences in how the test is performed.