What Is The Calcium Carbonate Content Of Limestone Vs Marble

Limestone vs marble.
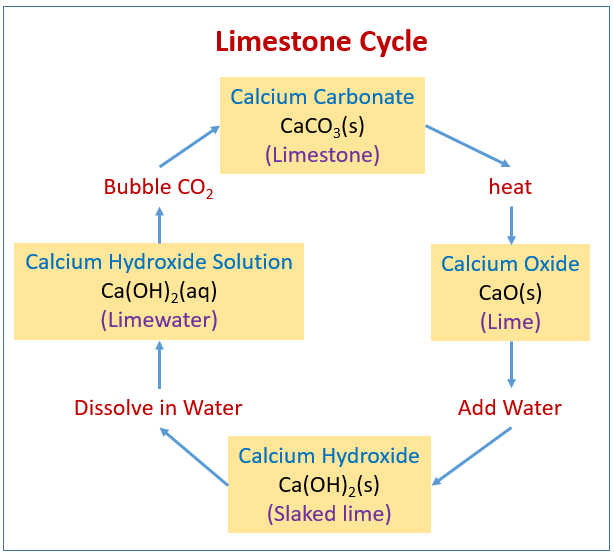
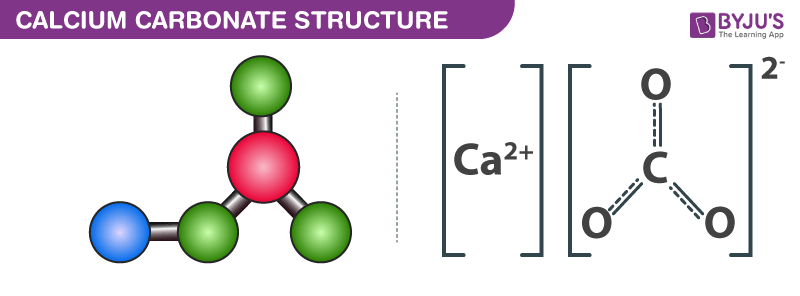
What is the calcium carbonate content of limestone vs marble. Calcium carbonate is a chemical compound with the formula ca co 3 it is a common substance found in rocks as the minerals calcite and aragonite most notably as limestone which is a type of sedimentary rock consisting mainly of calcite and is the main component of pearls and the shells of marine organisms snails and eggs calcium carbonate is the active ingredient in agricultural lime and. Though both limestone and marble consist of calcium carbonate they differ significantly in terms of appearance composition and application. The calcite form of calcium carbonate forms in all types of rock according to the minerals n more site. Limestone is a common sedimentary rock composed primarily of the calcium carbonate mineral calcite caco3.
It is found in marble limestone sandstone and shale. Limestone constitutes approximately 10 percent of the sedimentary rocks exposed on the earth s surface. Calcite is frequently phosphorescent in nature. Either limestone or marble may be used as the basis for crushed or ground calcium carbonate.
Either limestone or marble may be used as the basis for crushed or ground calcium carbonate. Often some of the calcium component is replaced by small amounts of iron magnesium or manganese. Even though their chemical nature is almost similar to each other there are many differences between limestone and marble in the way they originate and the physical characteristics they possess.