What Is Neutral Rubidium Electron Configuration

Rubidium complete electron configuration.
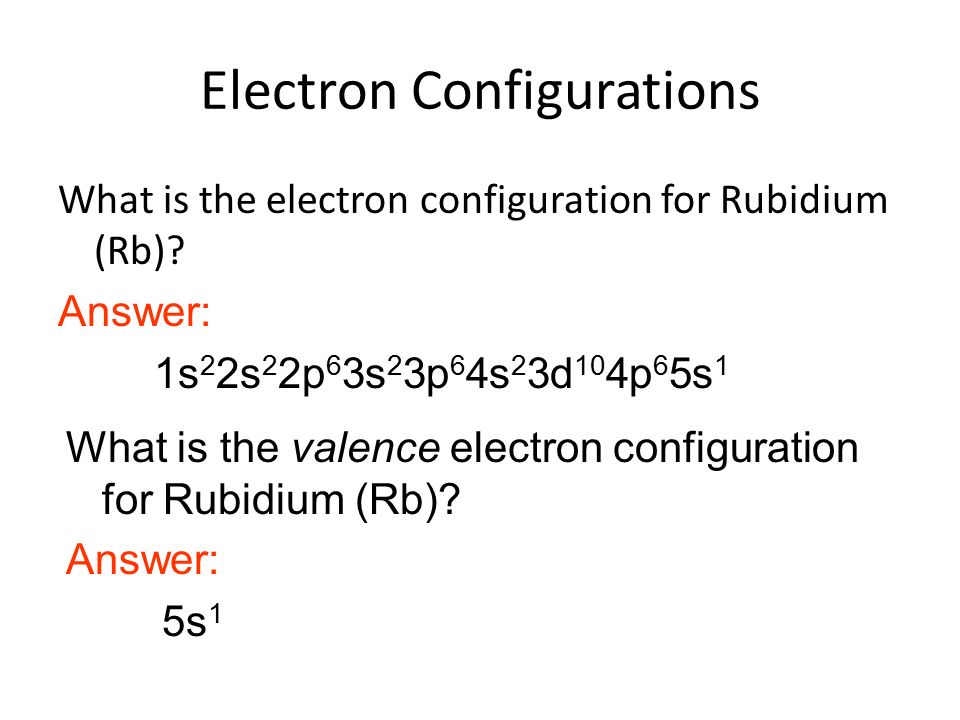
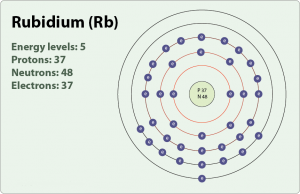
What is neutral rubidium electron configuration. Electron configuration chart for all elements in the periodic table. Rubidium atoms have 37 electrons and the shell structure is 2 8 18 8 1. 5s 1 and the term symbol is 2 s 1 2. Rubidium has a total of 37 electrons illustrated in the element s electron configuration of 1s2 2s2p6 3s2p6d10 4s2p6 5s1.
Usually obtained from lithium production. This list of electron configurations of elements contains all the elements in increasing order of atomic number. Each element has a unique atomic structure that is influenced by its electronic configuration which is the distribution of electrons across different orbitals of an atom. Rubidium rb has a 1 ion will have the same electron configuration as krypton kr because the 1 status means it has lost an electron.
The ground state electron configuration of ground state gaseous neutral rubidium is kr. This means part of the electron configuration has been replaced with the element symbol of the noble gas symbol. Rubidium has one valence electron which is located in the s orbital of the atom s fifth energy level. Rubidium overview rubidium complete electron configuration 1s2 2s2 2p6 3s2 3p6 4 s2 3 d10 4 p6 5 s1 abbreviated electron configuration kr 5s1 sources occurs abundantly but so widespread that production is limited.
Atomic symbol rb uses used as a catalyst photocells and vacuum and cathode ray tubes. To save room the configurations are in noble gas shorthand. There are 118 elements in the periodic table.