Sorin 3t Heater Cooler Cleaning

Sorin 3t heater cooler disinfection video.
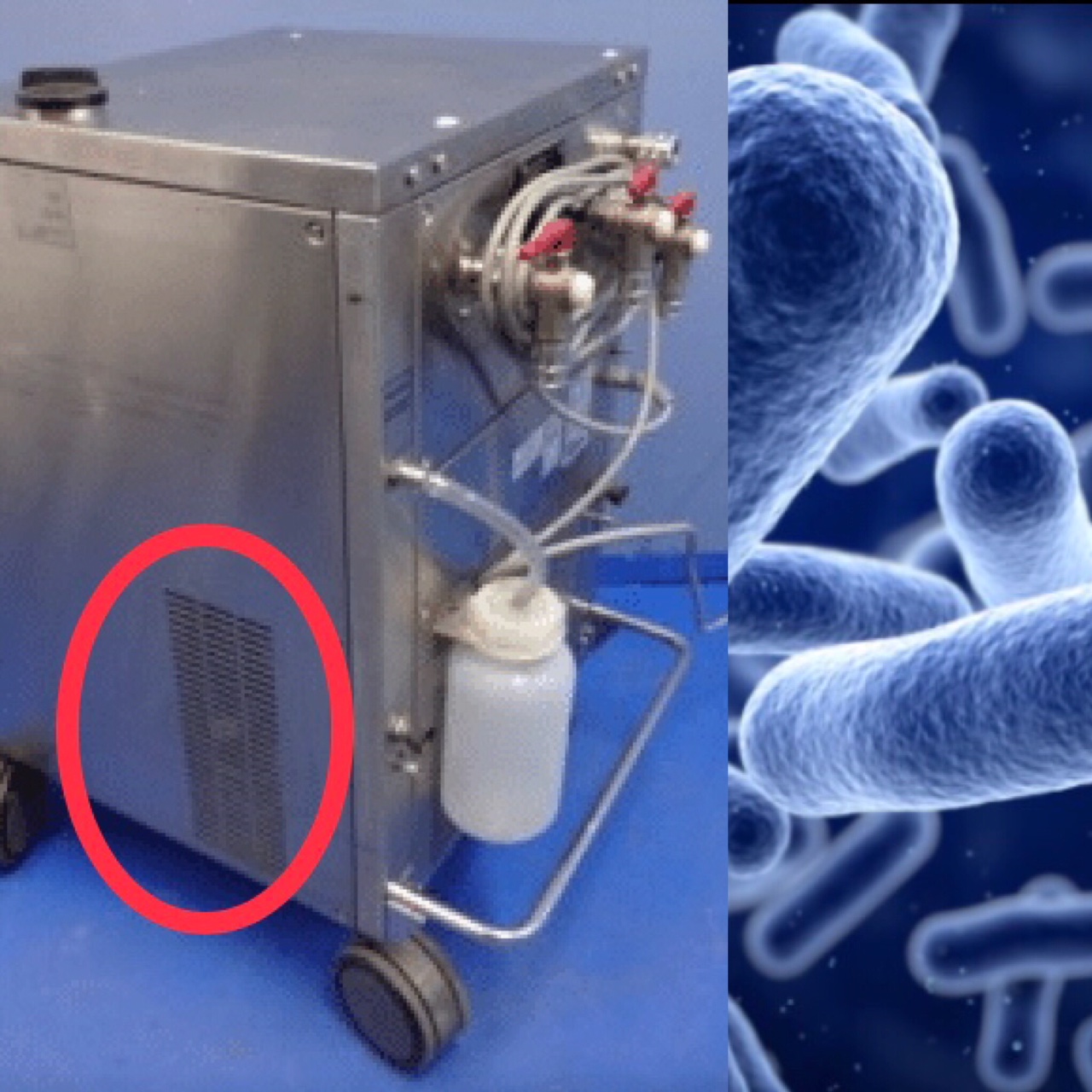
Sorin 3t heater cooler cleaning. Skip navigation sign in. Stöckert sorin s3 s5 c5 heart lung machine and or any other heart lung machine featuring a. Serial numbers 16s13187 16s14887 16s15335 16s15336 16s15337 and 16s11877. The stӧckert 3t heater cooler system 3t manufactured by livanova plc formerly sorin group deutschland gmbh is intended to provide temperature controlled water to 1 oxygenator heat.
Combining superior heating and cooling with worry free operation the mch series has made cardioquip the fastes. Sorin 3t heater cooler lawsuits. Heater cooler system 3t safety 2 2 cp ifu 16 xx xx usa 014 2 2 regulations and safety instructions 2 2 1 intended use in accordance with the applicable regulations the heater cooler is intended for use with a. Manufacturer of the modular cooler heater mch series of heater coolers.
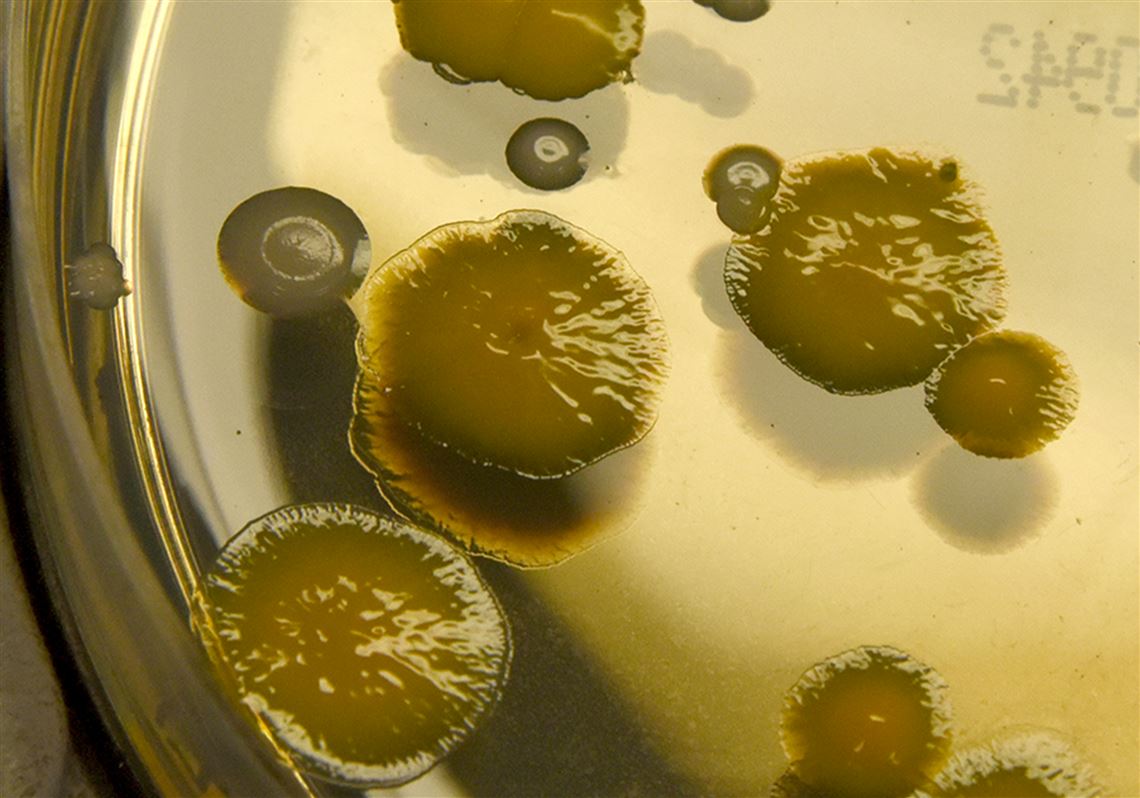
The sorin 3t heater cooler system used during various types of heart and lung surgeries can spread a dangerous bacteria during the procedures causing serious infections illnesses and even death. The stockert 3t device uses three tanks to circulate both heated and cooled water during invasive surgical procedures. Sorin stockert 3t heater cooler the sorin stockert 3t heater cooler is a device manufactured by livanova a medical device company formed by the merger of cyberonics and sorin s p a. The fda issued a new safety communication regarding the sorin 3t heater cooler system in february 2020.
Exhaust from the heater cooler system could disrupt the laminar space causing bacteria to enter the otherwise sterile area. In addition the fda cleared a new version of the heater cooler system 3t with changes to help reduce the risk of patient infections including updated labeling with validated cleaning and. The mch 1000 m and mch 1000 i. This video is unavailable.
The livanova 3t heater cooler system provides 3 circuits for fast and efficient heating and cooling to meet your patient and cardioplegia temperature needs. Compressor based cooling and 3 independent water tanks eliminate the need for ice and water supply in the operating room providing separate settings for warm cardioplegia cold. It includes several guidelines aimed at reducing the infection risk associated with this device. Lawsuits are currently being filed as a result of the infections caused by these products.